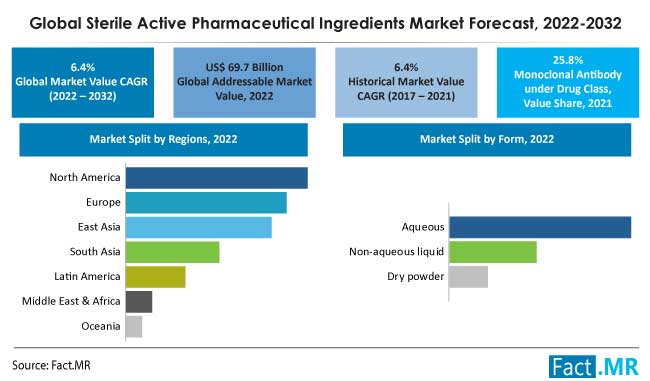
The market for sterile active pharmaceutical ingredients was estimated at US$ 69.7 billion in 2022, and by 2032, it is anticipated to grow at a global CAGR of 6.4% to reach US$ 130.2 billion.
By 2032, monoclonal antibodies will have more than 10% of the market for sterile active pharmaceutical ingredients (API) due to their expanding applications.
The study provides a comprehensive analysis of potential opportunities in several market categories for sterile active pharmaceutical ingredients from 2022 to 2032. Through several parts, including major players, competitive landscape, opportunity assessment, regional segmentation, and application/end-use analysis, it provides information on the market for sterile active pharmaceutical ingredients.
Click Here To get a Sample Report (Including Full TOC, Table & Figures):-https://www.factmr.com/connectus/sample?flag=S&rep_id=1702
The recent COVID-19 epidemic has forced key market participants, including regulators, business leaders, and investors from a wide range of nations, to continuously realign their approaches and objectives. These actions are required to address the COVID-19 pandemic-related setback and explore new corporate expansion opportunities. The study on the market for sterile active pharmaceutical ingredients provides insight into every tactic used by
Competitive Landscape
Prominent sterile active pharmaceutical ingredient manufacturers are Aurobindo Pharma Limited, Teva Pharmaceutical Industries Ltd., Corden Pharma GmbH, Dalton Pharma Services, Pfizer Inc, Sun Pharmaceutical Industries Ltd., Lonza Group, Albany Molecular Research Inc., Sanofi S.A., and Dr. Reddy’s Laboratories Ltd.
A growing need for parenteral medications, biologics, and ophthalmic solutions has manufacturers concentrating on growing their product lines related to sterile active pharmaceutical ingredients. Increasing production capacities is being accomplished by key industry participants using acquisition and merger tactics.
- In September 2019, ten23 health, a global contract development and manufacturing organization (CDMO), announced the completion of a new sterile manufacturing facility in Visp, Switzerland.
Fact.MR has provided detailed information about the price points of key manufacturers of sterile active pharmaceutical ingredients positioned across regions, sales growth, production capacity, and speculative technological expansion, in the recently published report.
Segmentation of Sterile Active Pharmaceutical Ingredients Industry Research
-
By Product Type :
- Monoclonal Antibodies
- Immunoglobulin
- Cytokines
- Insulin
- Peptide Hormones
- Blood Factors
- Peptide Antibiotics
- Vaccines
- Small Molecule Antibiotics
- Highly Potent Active Pharmaceutical Ingredients (HPAPI)
- Others
-
By Form :
- Aqueous
- Non-aqueous Liquid
- Dry Powder
-
By Region :
- North America
- Latin America
- Europe
- East Asia
- South Asia & Oceania
- MEA
This research provides thorough information on:
Along with a focus on future changes in the post-COVID era, significant regulations and standards are enacted by government authorities.
Studying strategies from rich and developing nations will help players recover from the COVID-19 outbreak by revealing what factors are crucial.
estimation of the size and proportions of significant product segments
research into various technologies that are crucial to the market for sterile active pharmaceutical ingredients and are driving demand
A summary of current and future research and development efforts by private Bacteriophage industry participants and public entities
a thorough examination of the financial repercussions that the COVID-19 epidemic would likely have in many global locales in the months to come
Contact:
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: sales@factmr.com